-
A motorcycle battery doesn’t last forever.
-
But what causes it to die?
-
We touch on why a battery degrades over time.
We touched on the basics about the motorcycle battery previously.
But rather than writing on battery maintenance alone, we feel that information on how a battery degrades gives a clearer picture on the type of maintenance required. Another note: We are covering lead acid batteries here as they are the most common compared to lithium-ion and lithium-iron-phosphate (LiFePO4). Regardless if your battery is “maintenance free” or otherwise, it’s considered a lead acid type as long as it’s filled with acid (as in sulfuric acid) as the electrolyte.
How does a battery work?
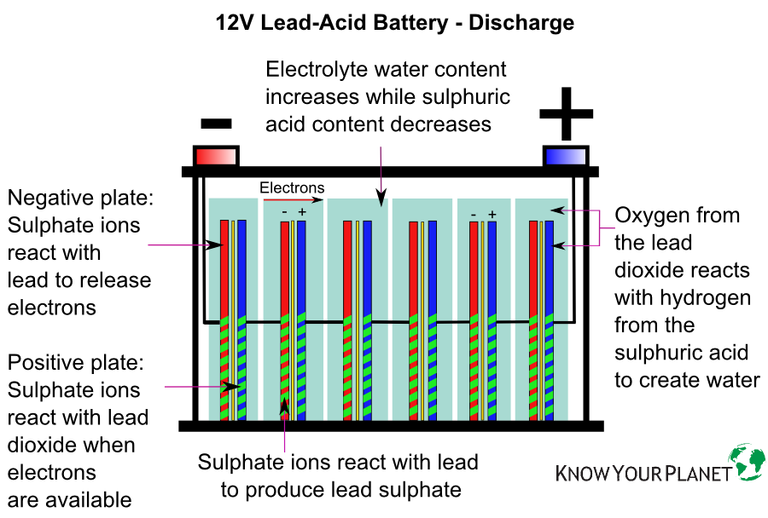
Here’s a recap on how a battery produces electrical power:
- The electrolyte solution contains charged ions, made up of sulphate (negatively charged) and hydrogen (positively charged).
- Placing an electrical load – starter motor, ECU, headlights, etc. – causes the sulphate ions to travel to the battery’s negative plates.
- The ions react with the plates’ active material to release electrons.
- These excess electrons move through the negative side of the battery to any device attached.
- The electrons travel back into the battery through the positive side.
- The movement of electrons is the battery’s direct current, measured in Ampere (A).
- The electrons then attach themselves to the positive plates.
How does a battery discharge?
- At the same time, the sulfuric acid breaks down.
- It means the electrolyte becomes less acid and more water.
- Lead sulphate coats the battery plates in each battery cell.
- The coated plates have less surface area to produce electrical energy.
- It causes the production of current to drop over time.
- If discharging continues, more lead sulphate is deposited on the plates.
- Eventually, the chemical process that produces current stops.
- A battery may not recover despite how long it’s charge if there’s heavy sulfation.
Self-discharge
- All batteries self-discharge over their lifetime, even if they’re not attached to any circuit or load.
- Sulfation occurs as long as the battery’s state of charge is below 100%.
- The rate depends on battery type and ambient temperature.
- Sulfation occurs if the battery is overcharged, undercharged or left discharged for just a few days.
- Smaller lead acid batteries like those in motorcycles sulphate faster.
- Using or storing batteries above 24 oC (75 oF) accelerates self-discharge and increases sulfation.
- The discharge and sulfation rate doubles with the increase of every 10 o
What are the reasons for self-discharge?
- Short trips i.e. within 25 to 30 km may not build enough charge.
- So is occasional use i.e. once or twice a week.
- Parasitic discharge i.e. motorcycle’s electronics that don’t fully turn off.
- Problems in the bike’s electrical system.
- Problems with the charging coil (stator).
We would like to add here that a motorcycle’s charging system may not charge the battery the way it likes to be charged. This could lead to certain plates being undercharged resulting in sulfation and dead cells, eventually.
What does all this mean?
This is why batteries do not last very long in our climate. A survey in conducted in the United States showed that 85% of batteries do not last up to 4 years, and the best case was just below 3 years.
It must be charged sufficiently to prevent it from dropping below 12.4 Volts.
What do we need to do then?
So, batteries aren’t exactly “maintenance free” and plug-and-play as we’d like, hence proper battery maintenance must be carried out. But first, consider fitting a voltmeter on your bike for you to keep an eye on the bike’s charging rate and battery’s health.
Keeping your battery charged up avoids that hassle of the bike refusing to start. Imagine if happens at night when all the shops are closed or you’re kilometres away from the nearest town. Worse still, you need to fork out some emergency cash.
While we can’t disagree that replacing the battery every two years solves this problem, I’ve personally used my batteries more than 3 years before replacing them, thus saving me a lot of money in the long run. I did this by using a smart charger.
A smart charger which can evaluate the battery’s state and carry out the appropriate charging strategy/strategies. These chargers pump in a certain amount of Amperage to reverse the sulfation, get it up to full capacity and finally maintain that capacity with a trickle charge.
We’ll stop here for this instalment. We’ll look at battery maintenance and smart chargers in detail in the next article.